The Pioneers inDental Implant & E.rhBMP-2
Cowellmedi is a specialized dental implant company that developed Korea's first dental implant in 1994.
It took the lead in dental implant development and successfully localized the technology when only foreign implants were available. With a strong commitment to providing patients with safe and sustainable dental implants that closely resemble natural teeth, Cowellmedi has consistently invested in delivering innovative solutions.
Furthermore, to address the limitations of existing implants, Cowellmedi has actively invested in research and development, resulting in the development of excellent dental implants and the provision of predictable clinical solutions. As a result, Cowellmedi has obtained numerous international certifications, including FDA(US), CE(Europe), MHRA(UK), and HC(Canada). And Cowellmedi products and services are available over 70 countries worldwide.
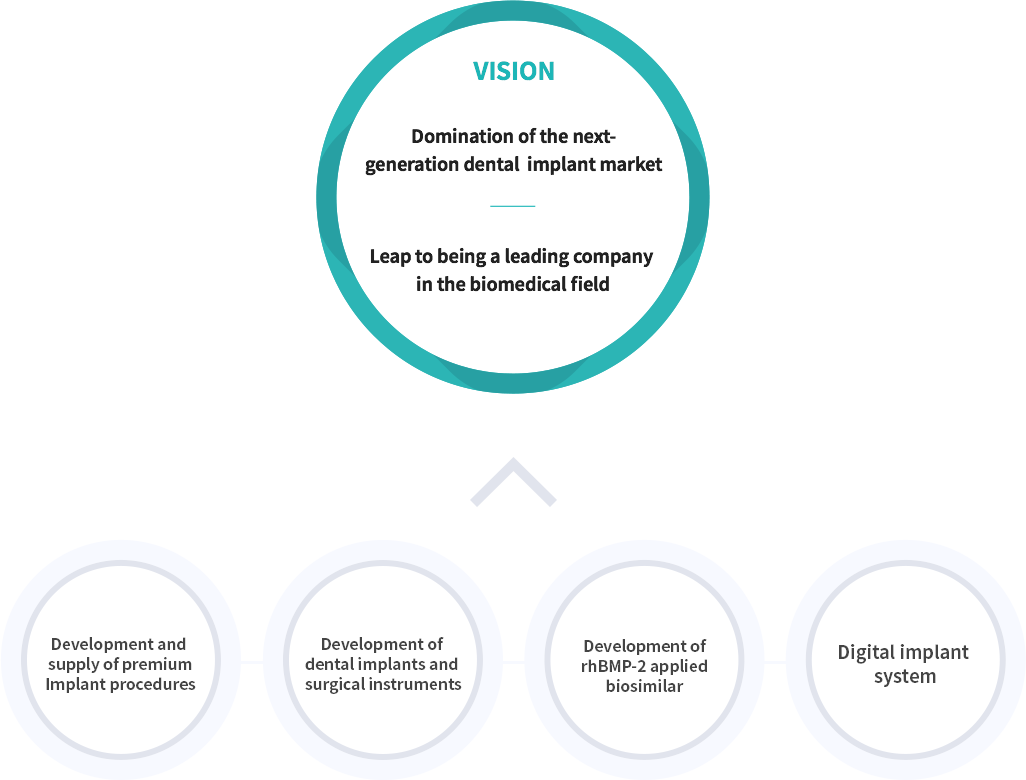
In 2010, Cowellmedi achieved a groundbreaking milestone by successfully developing and commercializing E.rhBMP-2-based bone grafting materials, becoming the world's first company to do so. This achievement has allowed Cowellmedi to provide patients with more predictable treatment effects and establish a leading position in the biomedical industry.
Building upon the past 30 years of accomplishments and experience, Cowellmedi will make every effort to develop another innovation providing patients with optimal treatment experience, as it is already happening.