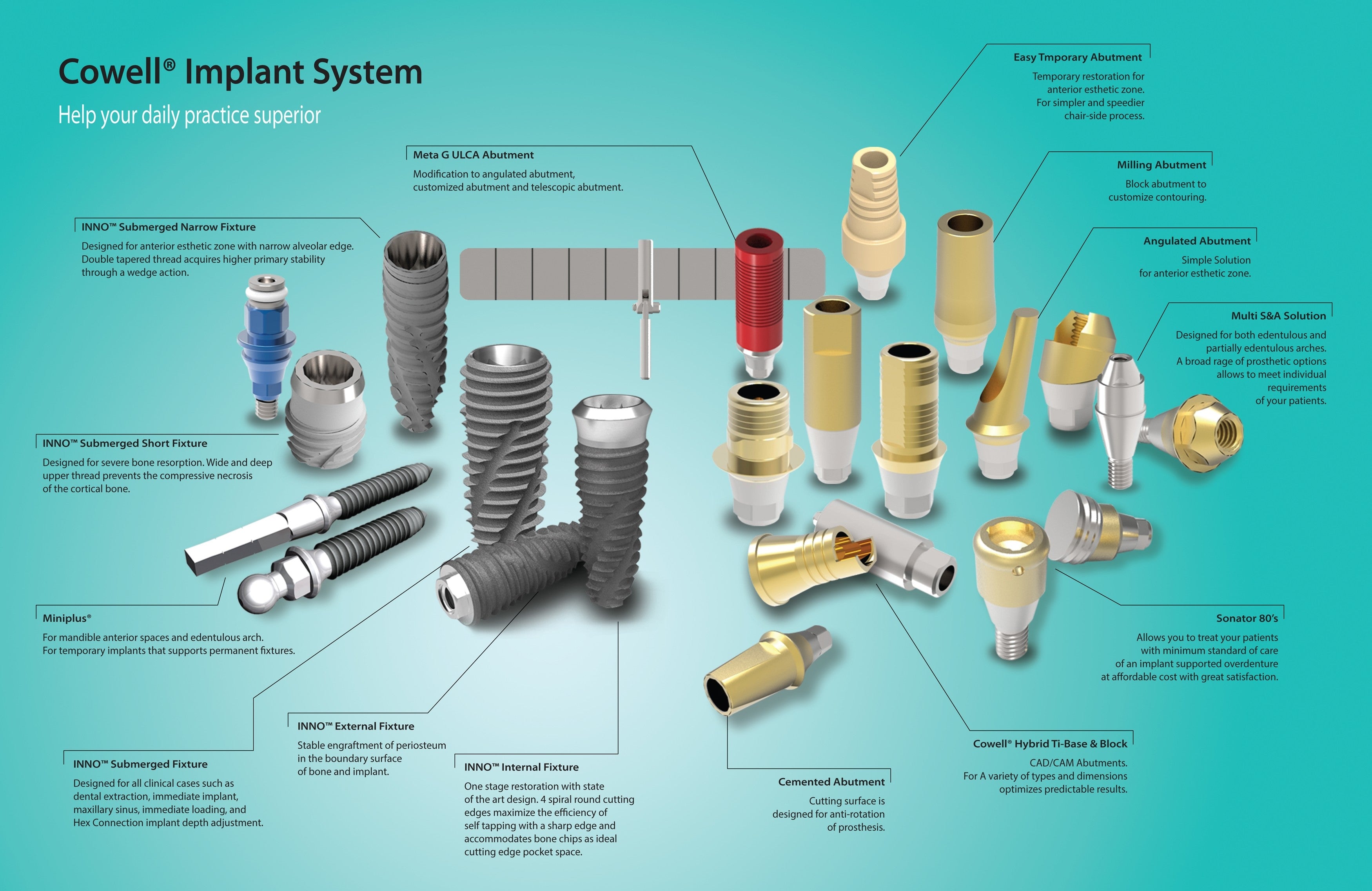
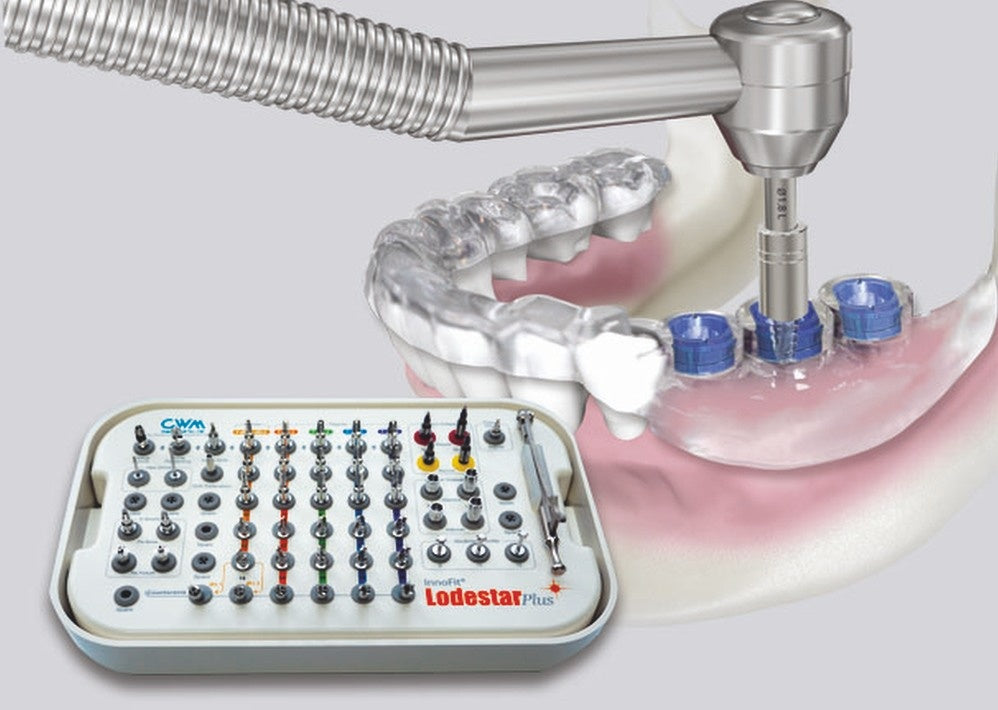
The Pioneers inDental Implant & E.rhBMP-2
Cowellmedi is a specialized dental implant company that developed Korea's first dental implant in 1994.
It took the lead in dental implant development and successfully localized the technology when only foreign implants were available. With a strong commitment to providing patients with safe and sustainable dental implants that closely resemble natural teeth, Cowellmedi has consistently invested in delivering innovative solutions.
Furthermore, to address the limitations of existing implants, Cowellmedi has actively invested in research and development, resulting in the development of excellent dental implants and the provision of predictable clinical solutions. As a result, Cowellmedi has obtained numerous international certifications, including FDA(US), CE(Europe), MHRA(UK), and HC(Canada). And Cowellmedi products and services are available over 70 countries worldwide.
In 2010, Cowellmedi achieved a groundbreaking milestone by successfully developing and commercializing E.rhBMP-2-based bone grafting materials, becoming the world's first company to do so. This achievement has allowed Cowellmedi to provide patients with more predictable treatment effects and establish a leading position in the biomedical industry.
Building upon the past 30 years of accomplishments and experience, Cowellmedi will make every effort to develop another innovation providing patients with optimal treatment experience, as it is already happening.
Hyunmyung Choe
CEO, Cowellmedi Co., Ltd.
Mission
Dental specialty company that enhances
the quality of human lives by realizing
their desire to pursue beauty and youth.
Vision
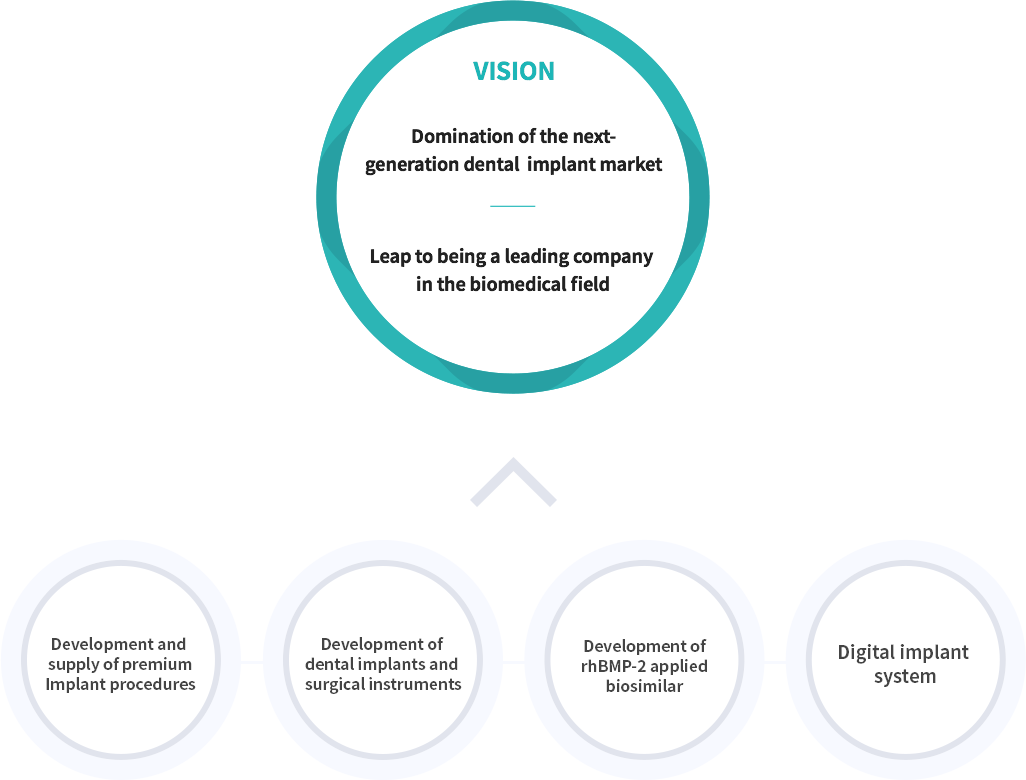
Value
A daring spirit as a
“Research Company” that focuses on R&D.
The first Dental Implant in Korea.The history of Cowellmedi at a glance.
1994 ~ 1999
-
1999
-
12Entered an industrial-academic cooperation agreement with Pusan National University’s Oral and Biotechnology Research Center.
-
11Obtained medical device manufacturing license(MFDS; Ministry of Food and Drug Safety).
Obtained medical device manufacturing approval for its BIOPLANT Implant System (MFDS).
-
1998
-
09Asrahi Medical founded (CEO : Soohong Kim D.D.S. Ph.D).
-
1994
-
03Successfully developed localization dental implants for the first time in Korea (Soohong Kim D.D.S. Ph.D).
2000 ~ 2009


-
2009
-
01Obtained approval for clinical test plan for rhBMP-2 bone graft material(COWELLBMP)
(MFDS; Ministry of Food and Drug Safety).
-
2008
-
05Completed clinical trials for rhBMP-2 bone graft material(COWELLBMP)
(Korea University Guro Hospital, Seoul National University Bundang Hospital).
-
01Began selling rhBMP-2 research reagents.
Developed and began selling Sinus Lift Kit.
Launched the sales of its products for training North Korean dentists
(Dental Implant Products, Surgical Kits and Dental Engines).
-
2007
-
12Opened Seoul Branch and launched direct sales operation.
-
07Obtained a patent for rhBMP-2 Coated Implant.
-
05Applied for a US patent for rhBMP-2 Coated Implant.
-
2006
-
12Selected as an Excellent Entrepreneur (Busan Metropolitan City).
Selected as a Leading Company in a Strategic Industry (Busan Metropolitan City).
-
10Received the Excellent Venture Entrepreneur Award (Busan Metropolitan City).
Established Cowellmedi Tissue Engineering Institute for Growth Factors.
-
05Designated as a Family Firm (IBK; Industrial Bank if Korea).
-
2005
-
12Obtained ISO13485 Certificate.
-
03Obtained a GMP Certificate (KGMP; Korea Good Manufacturing Practice).
-
2004
-
09Established R&D Center.
Received an Excellent Small & Medium Business Award (SMBA).
CEO Dr. Soohong Kim D.D.S. Ph.D received the Bronze Tower Order of Industrial.
Service Merit at the 5th Innovative Technology Show.
Obtained USFDA approval for BIOPLANT Implant System.
-
04Selected as a Superior Technology Company (KTFC; The Korea Technology Finance Corporation).
-
03Received an Excellent Small & Medium Business Award (SMBA).
-
2003
-
03Selected as an INNOBIZ Company (SMBA).
-
01Selected as an Export-oriented Company (SMBA).
-
2002
-
10Selected as a Promising Export Firm (SMBA).
-
2001
-
04Selected as an Innovative Technology Development Company (SMBA).
-
2000
-
11Designated as a Venture Company (SMBA).
-
10Selected as a Promising Export Firm (SMBA; Small & Medium Business Administration).
-
09Obtained ISO-9001 Certification.
-
05Converted Corporation to COWELLMEDI Co., Ltd.
2020 ~ Today
-
2021
-
08Obtained Vietnam’s approval and certification for INNO Impalnt System and Cowell BMP.
Obtained Medical Device License in Canada (Health Canada).
-
07Obtained China’s approval and certification for INNO Impalnt System (NMPA).
-
2020
-
09Obtained MDSAP (Medical Device Single Audit Program) certification (TUV).
-
06Global Hidden Champion by Ministry of SMEs.






 USFDA Certification
USFDA Certification
 European Quality
European Quality ISO 13485 Certification
ISO 13485 Certification
 Ministry of Food and
Ministry of Food and